
Degrad Plus
Uncover drug degradation pathways, API-excipient incompatibilities, and stability risks through hybrid quantum-AI reactivity calculations meets expert knowledge bases.
Foresee API Failures: Instant ICH Stress Predictions


FDA 21 CFR Part 11 and ANVISA RDC 658/2022 compliant from day one.
Experimental Expenses
Traditional forced degradation studies require intensive lab protocols, high-cost reference standards, and repetitive method optimization.
These approaches often detect degradation pathways only after initial investments, triggering downstream analytical challenges and escalating hidden costs in early pharmaceutical development stages.
Formulation Lags
Compliance risks
Unknown peaks, overlapping degradants, and mass balance deviations in stability studies often demand months of extra analytical work to identify and separate impurities.
This uncertainty delays regulatory submissions, complicates impurity profiling, and increases the risk of compliance issues throughout pharmaceutical development.
Up to 40% of formulations experience stability failures after scale-up due to API-excipient incompatibilities. Each reformulation cycle can add significant delays, often pushing product launches back by several months.
These setbacks disrupt project timelines and jeopardize competitive advantage in bringing new products or therapies to market.
Stringent regulations from FDA, EMA (ICH Q1A/B), and ANVISA's RDC 964/2025 require complete degradant profiling before forced degradation studies.
Incomplete documentation or missing impurity assessments create submission risks, portfolio reassessment demands, and delays in product approval across markets.












Analytical Uncertainty
Traditional stability approaches react to problems after they appear. By then, you've already lost time, budget, and competitive advantage.
The hidden costs of stability failures









Replace broad trial‑and‑error studies with focused experiments guided by predicted high‑risk pathways.
Reduce delays from late stability failures, keeping projects on schedule instead of restarting after scale‑up surprises.
Why teams choose Degrad Plus?
Save months on stability work
Cut unnecessary experimental costs
Avoid running dozens of low‑value stress conditions and methods by concentrating spend on degradants that truly matter.
Reduce spend on reference standards, long analytical investigations, and repeated reformulations driven by unexpected impurities.
Compare excipients and conditions in silico to prioritize more robust formulations before investing in lab work.
Channel budget and team time into candidates with the highest chance of long‑term stability and successful launch.
Design stronger formulations from day one




Increase confidence with regulators and stakeholders
Support stability and impurity justifications with transparent, mechanism‑based reasoning instead of ad‑hoc explanations.
Give R&D, QA, and regulatory teams a shared risk picture, reducing rework, internal debate, and the cost of late documentation fixes.

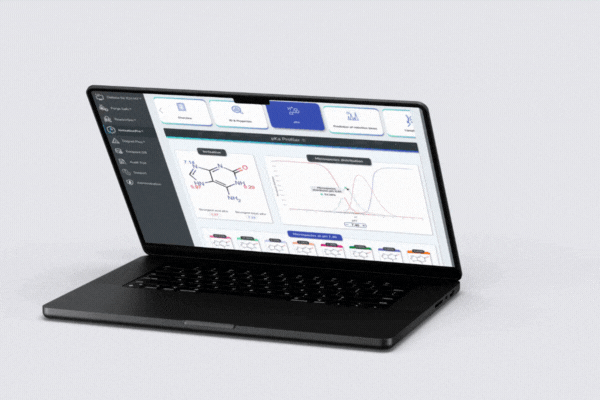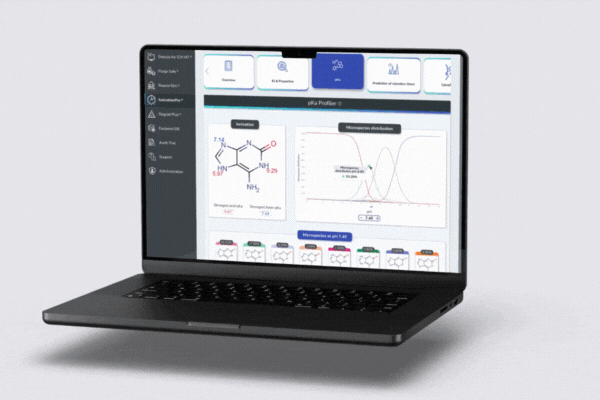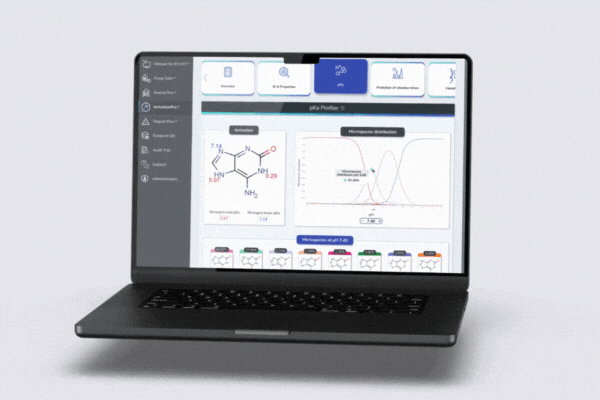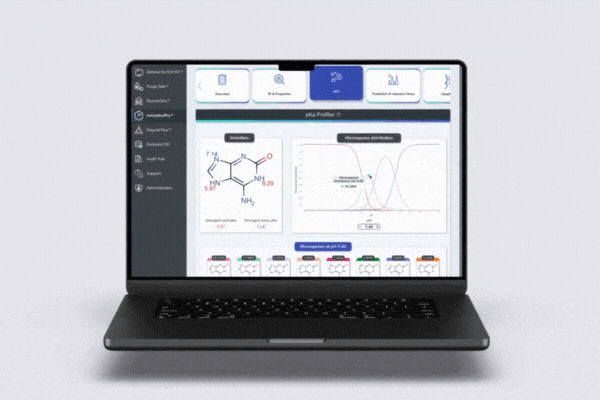
Analysis setup
Select excipients from our comprehensive database: polymers, salts, generics, and impurity profiles (115+ validated).
Set up one-click ICH conditions including pH, temperature, light, oxygen, and humidity, or customize your own analysis parameters.
Define the number of steps, score thresholds, maximum degradants, and mass details for targeted results.
Choose whether to display duplicates, interactions, and identified compounds within the degradation pathway tree.
Predictive Insight: The Degrad Plus Engine
Achieve deep visibility into degradation pathways and formulation robustness, purpose-built for pharmaceutical and chemical R&D.
Built on hybrid AI models combining knowledge-based rules and quantum mechanical calculations, Degrad Plus offers the predictive clarity development teams need to anticipate degradation risks and streamline formulation decisions before experimental testing.


Comprehensive Degradation Mechanism Coverage


Interactive Degradation Maps
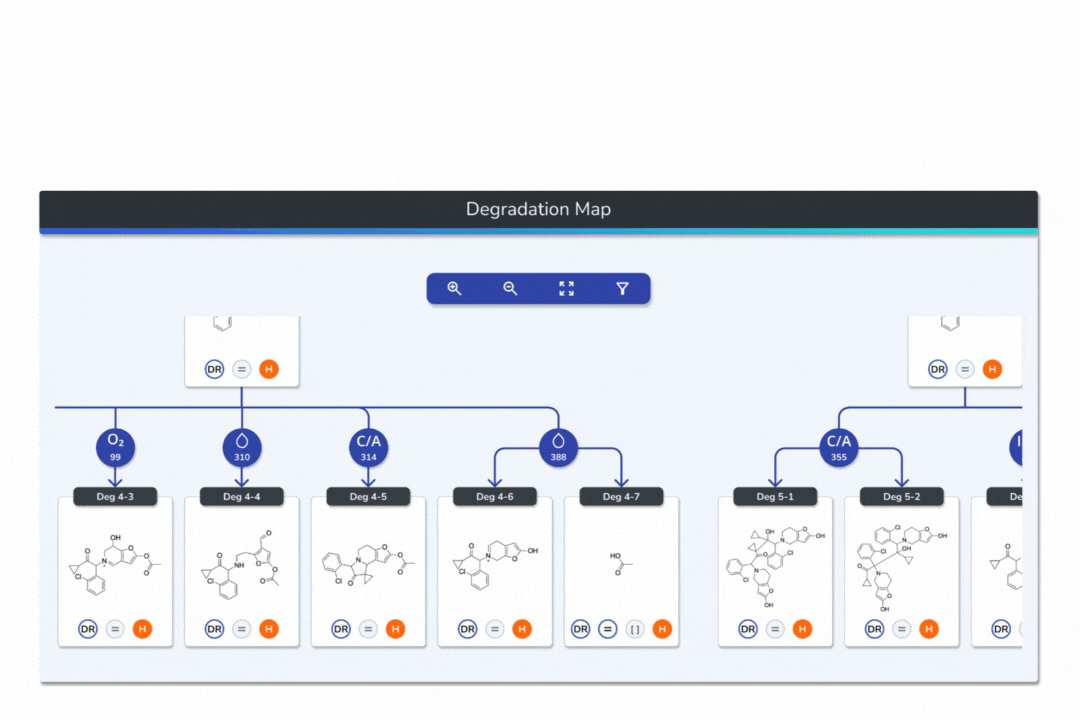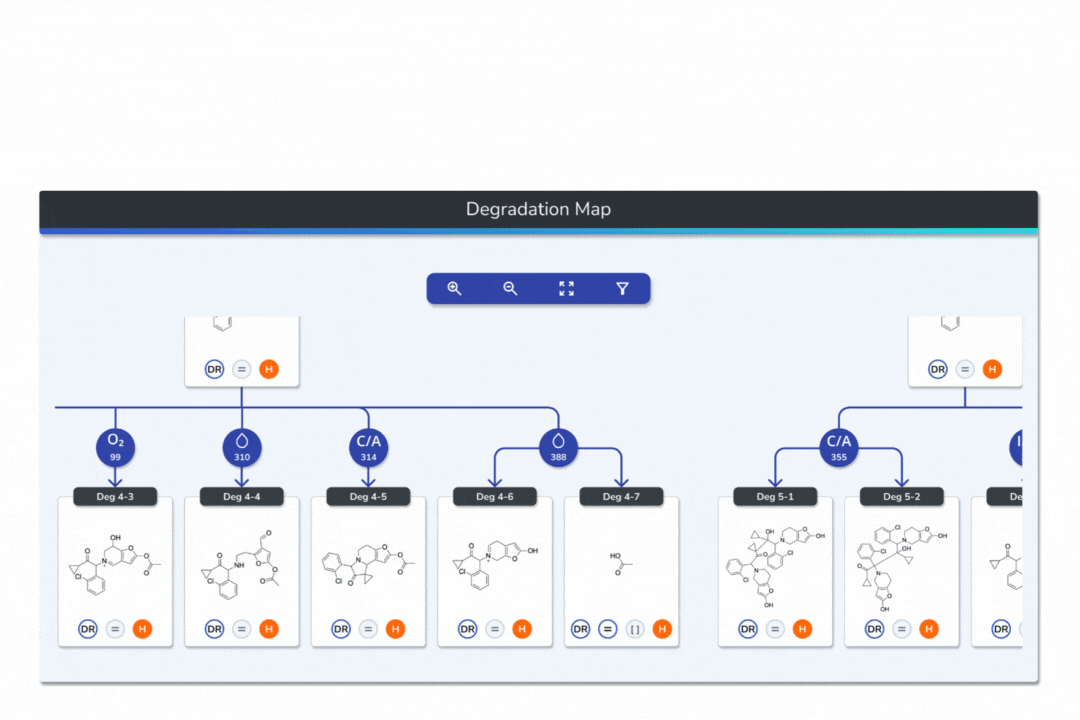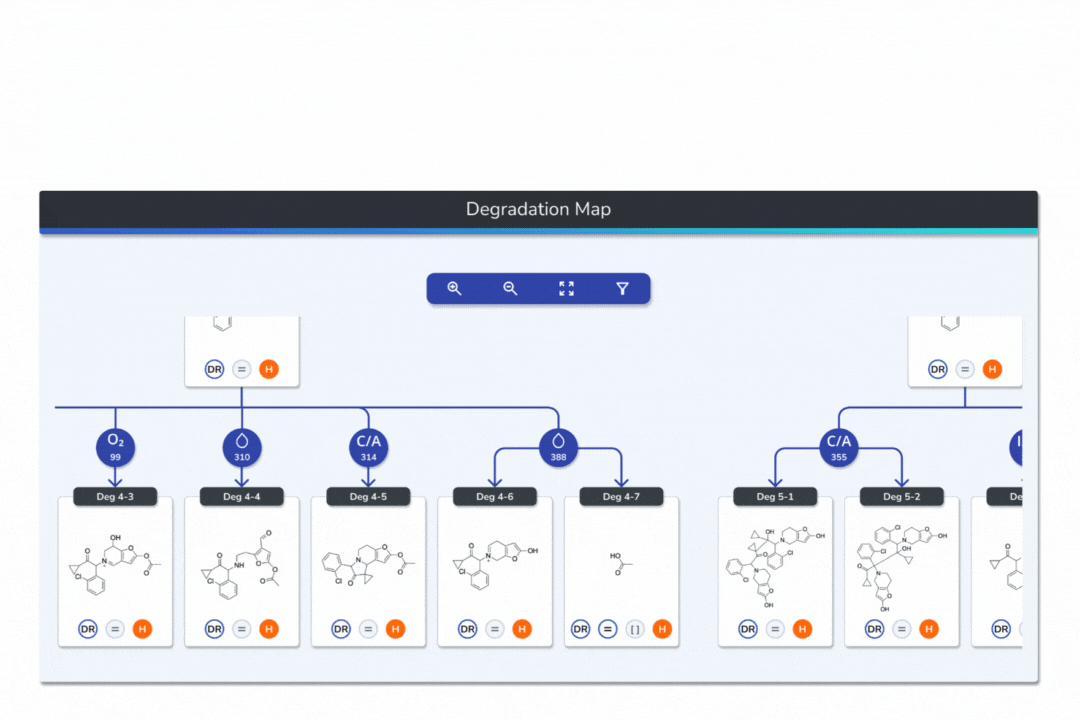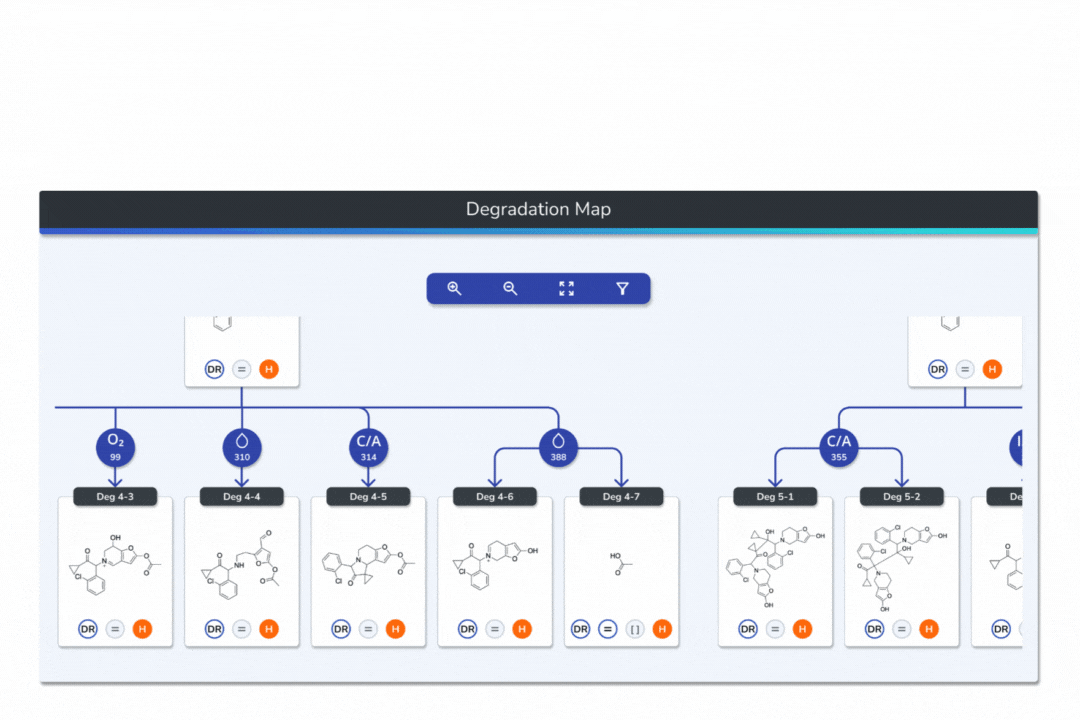

Visualize your API and predicted degradants in an interactive tree graph, each node scored by probability for fast prioritization.
Explore every transformation in the degradation pathway from parent to product.
Smart tools: change analysis conditions, filter scores, search mass/formula, highlight API-excipient interactions.
Export graphical degradation schemes for documentation or further analysis.
Degradant Detail Cards
Each degradant is shown as an interactive card with full chemical data: molecule image, formula, SMILES and mass information.
Interaction Identification: clearly identify Parent–Parent, Parent–Excipient, Parent–Degradant, and Degradant–Degradant interaction pathways.
Easily detect common degradation products formed through multiple routes.
Instantly identify compounds referenced in Chemical Databases, Pharmacopoeias, Cataloged Impurities and Regulatory Documents.
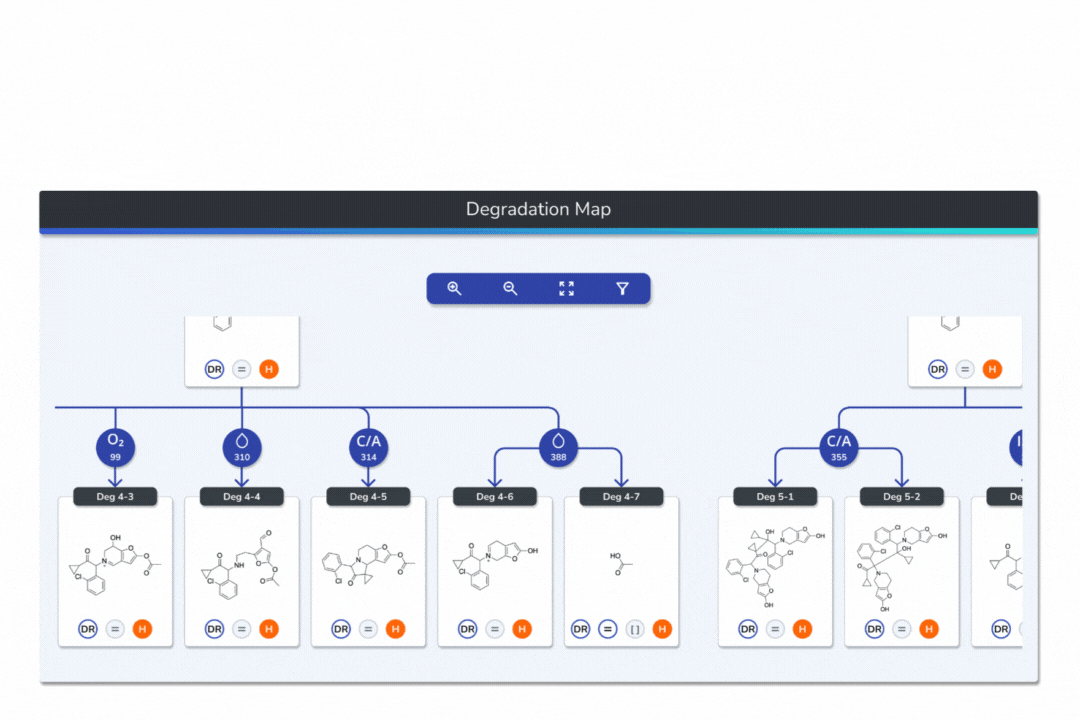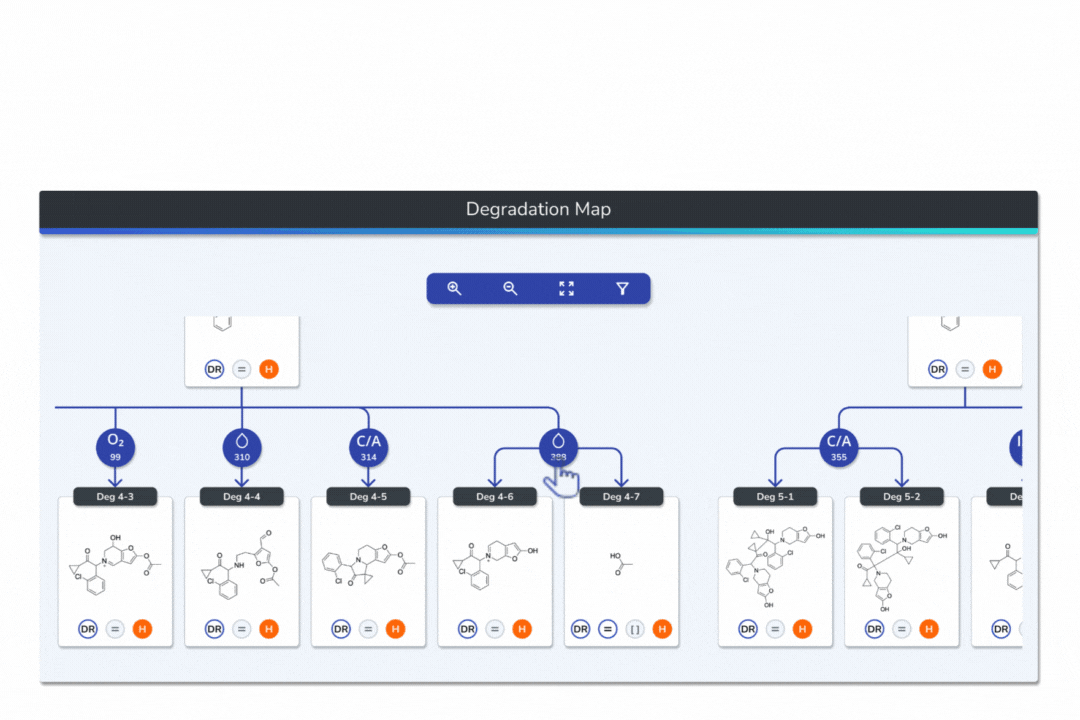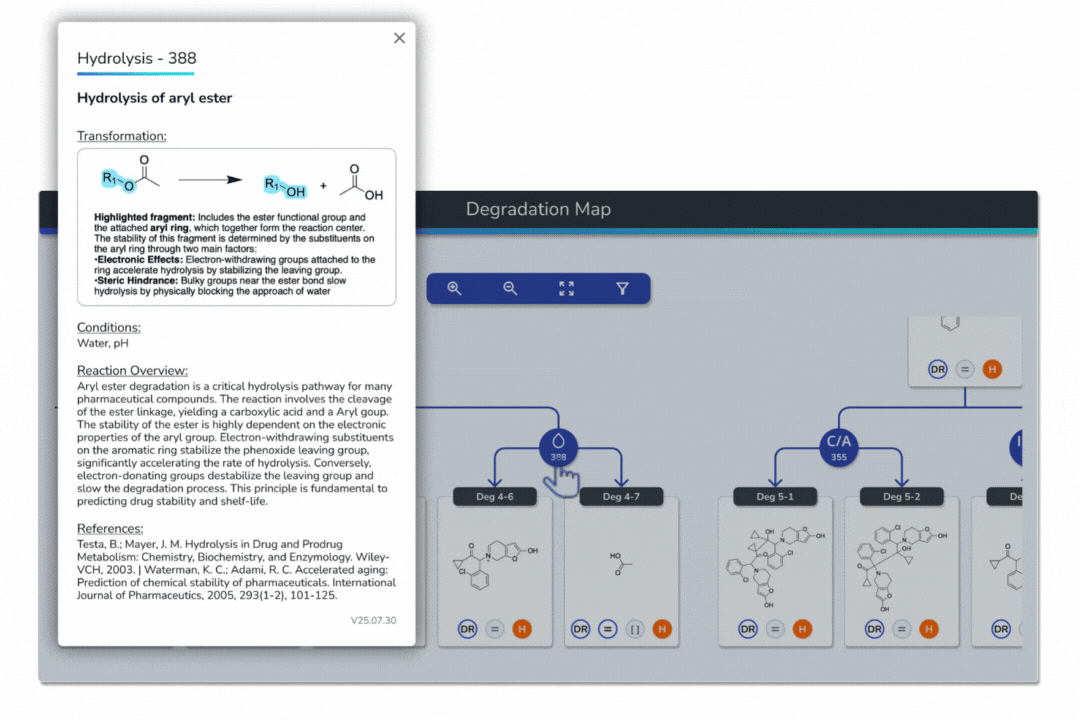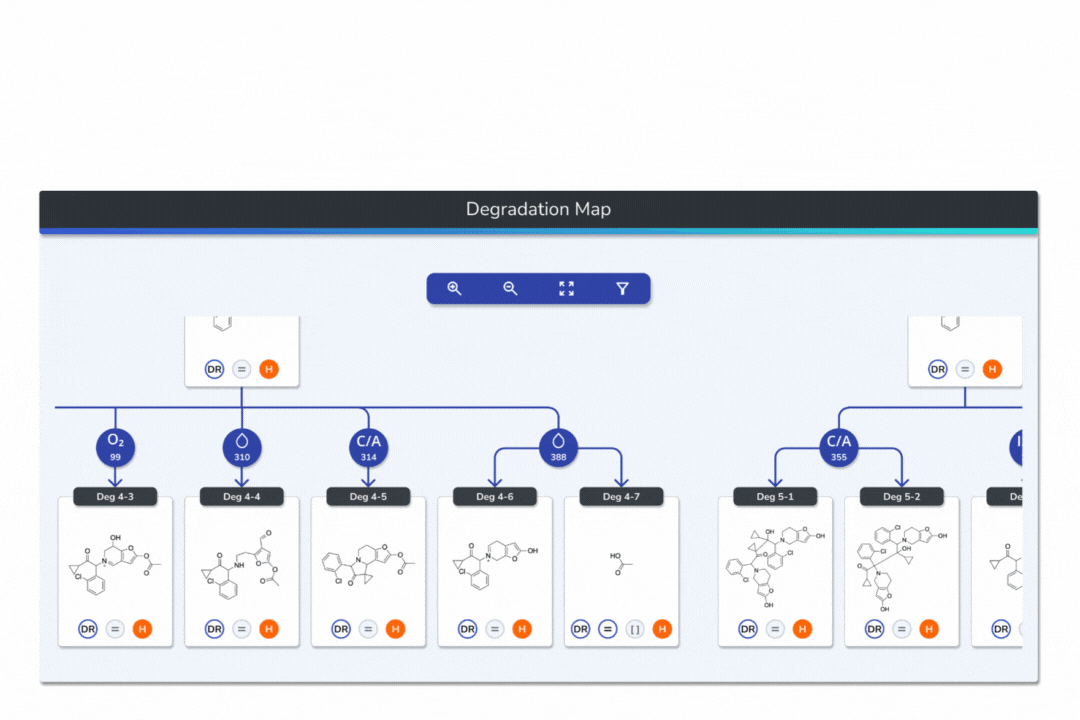

Get complete explanations for each transformation, with highlighted reaction fragments.
Check reaction conditions required for each pathway.
Read concise reaction overviews with mechanistic insights.
Access supporting literature and references directly in each modal.
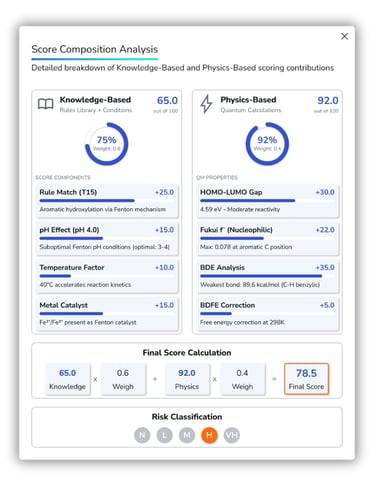
Degradant Formation Risk Scoring
Five formation risk levels, from Negligible to Very High, calculated by combining knowledge and physics scores into a single probability value for each degradant.
Knowledge-Based Score: Uses a proprietary library to generate condition-aware scores that adjust for pH and temperature, guiding the design of targeted stress studies and formulation strategies.
Physics-Based Score: Quantifies intrinsic reactivity using quantum calculations, HOMO–LUMO/Fukui analysis, and AI-predicted bond dissociation energies.


Regulatory-Grade Documentation
Generate immutable PDF reports with embedded QR codes.
Supports FDA 21 CFR Part 11, ANVISA RDC 658/2022, and GAMP 5 compliance requirements.
Comprehensive audit trails track all user actions and data modifications, ensuring data integrity and traceability for regulatory submissions.
Master your degradation profile for scientifically robust, gap-free submissions




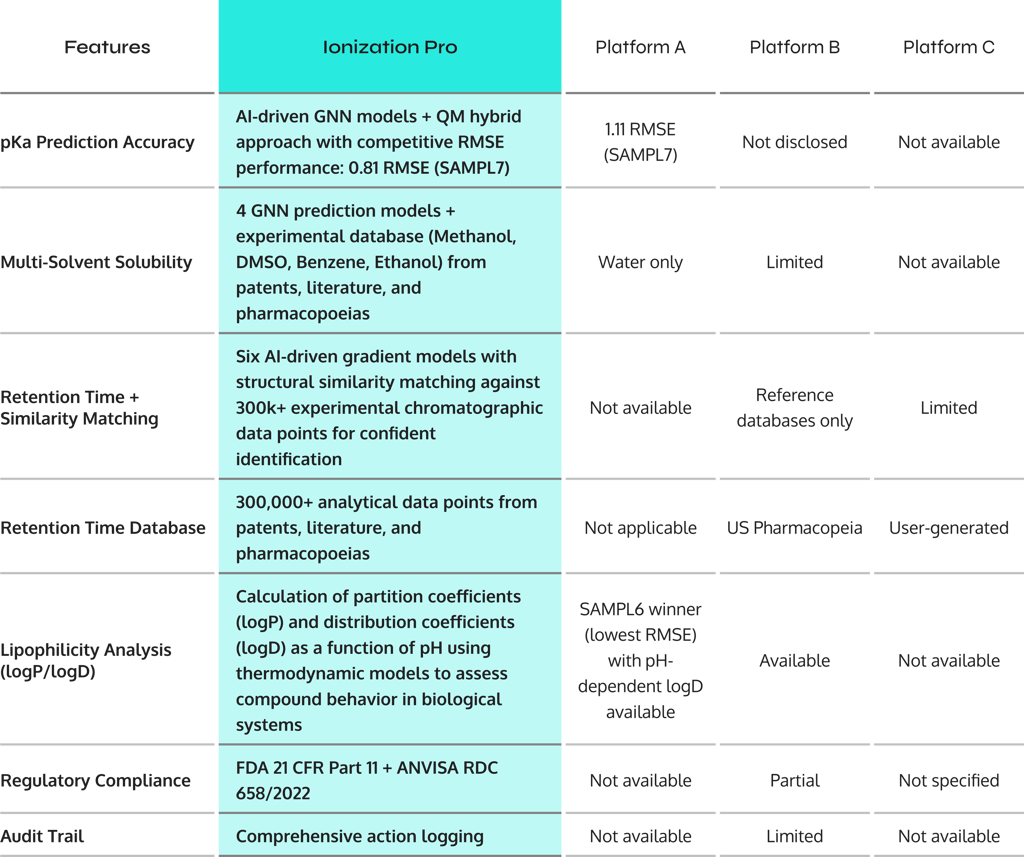
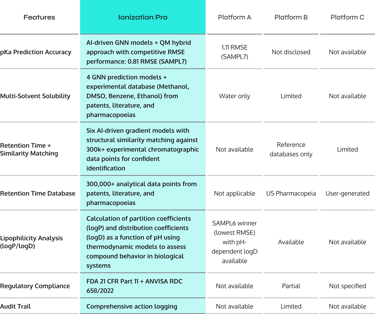
Why Degrad Plus outperforms traditional solutions



From Structure to Submission-Ready Insights in 5 Steps

From Structure to Submission-Ready Insights in 5 Steps
Need more information? View Degrad Plus FAQ

Still have questions? Connect with our Experts.
If your specific scientific or regulatory questions remain unanswered, our team is ready to help.
Our Masters and Ph.D. experts can provide the detailed guidance you need or show you how Degrad Plus empowers you to predict, prioritize, and justify every degradation pathway.


